Press Releases
- Revenue increases 30% to $6.1 million, Adjusted EBITDA2 increases 40% to $4.1 million
- EPS increases 450% to $0.11 (CDN$0.141)
- Epuris revenue grows 63%, Absorica revenue grows 25%
- All figures in U.S. dollars, unless otherwise noted
OAKVILLE, ON, Aug. 12, 2021 /CNW/ - Cipher Pharmaceuticals Inc. (TSX: CPH) ("Cipher" or "the Company") today announced its financial and operating results for the three and six-months ended June 30, 2021. Unless otherwise noted, all figures are in U.S. dollars.
Q2 2021 Financial Highlights
(All figures in U.S. dollars, compared to Q2 2020, unless otherwise noted)
- Total revenue increased 30% to $6.1 million compared to $4.7 million
- SG&A decreased 10.3% to $1.2 million compared to $1.3 million
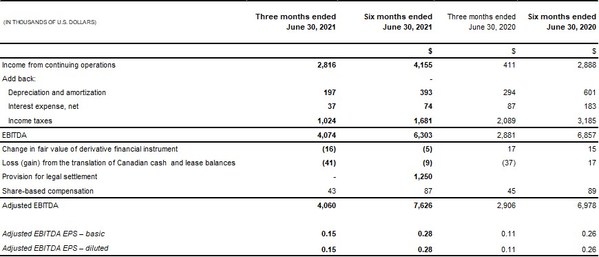
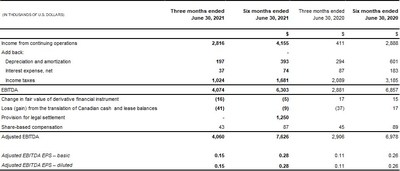
- Adjusted EBITDA increased 40% to $4.1 million compared to $2.9 million
- Earnings per common share increased 450% to $0.11 compared to $0.02
- As at June 30, 2021 the company had $16.1 (CDN$19.9) million in cash or $0.60 per share (CDN$0.74)
- Normal Course Issuer Bid – 631,000 shares repurchased and cancelled to the end of June 30, 2021
Management Commentary
Craig Mull, Interim CEO commented, "Our second quarter results demonstrated strong sequential and year-over-year growth in revenue, EBITDA and earnings per share, driven by growth in our license and product portfolios. In addition, we generated $7.6 million in cash from operating activities in the six-months ending June 30, 2021, ending the quarter with a strong balance sheet, and placing us in an excellent position as we continue to assess growth opportunities and maximize income generated from our distribution agreements."
"During the second quarter we launched Absorica AG with our marketing partner Sun Pharmaceutical Industries, Inc. ("Sun Pharma"). We believe that this will broaden Cipher's isotretinoin portfolio and ensure we have products to serve each segment of this market and maximize the value of this portfolio. The U.S isotretinoin prescription market increased by 18.2% in the six-month period ended June 30, 2021, which helped drive growth in our licensing revenue during the second quarter, as we were first to market with an authorized generic version of Absorica®. Although it is still early, it is our belief that the lower priced generic may have driven an expansion of the overall market."
"Currently, in our isotretinoin portfolio, Cipher is receiving royalties from Sun Pharma for the branded product, Absorica, the authorized generic, as well as ABSORICA LD®," noted Craig Mull, Interim CEO of Cipher. "We are confident that working with Sun Pharma is the right economic decision for Cipher and is consistent with our overall strategy to maximize the value of the isotretinoin portfolio," added Mr. Mull.
Outlook
Cipher anticipates several key milestones in 2021 that will continue to enhance long term value, including:
- Full-year benefit of the cost reduction plan and continued profitability and strong cashflow
- Improved profitability of our hospital business through a distribution agreement with Verity
- Advancement of our key development programs including: refinements to the MOB-015 program for nail fungus with Moberg with a possible expansion of territories; completion of proof-of-concept studies for our tattoo removal program; negotiation of development agreements for three products with our development partner, Galephar.
- Normal Course Issuer Bid – 631,000 shares repurchased and cancelled to the end of June 30, 2021 Management expects to renew the NCIB program when the current program expires on August 12, 2021
- Selectively pursuing product and business acquisitions in a prudent manner with a focus on high growth potential and near-term profitability
Q2 2021 Financial Review
(All figures are in U.S. dollars)
Total net revenue increased 30% to $6.1 million for Q2 2021, compared to $4.7 million for Q2 2020.
Licensing revenue increased by $0.1 million to $2.8 million for the quarter compared to $2.7 million for the three months ended June 30, 2020.
Licensing revenue from Absorica in the U.S. was $2.4 million for the quarter, an increase of $0.5 million or 25% compared to $1.9 million for the three months ended June 30, 2020. Absorica and the authorized generic version of Absorica's market share for the three months ended June 30, 2021 was approximately 4.2% compared to 6.5% for the three months ended June 30, 2020 according to Symphony Health. Market share including Sun Pharma's Absorica LD® was approximately 6.2%.
Licensing revenue from Lipofen and the authorized generic version of Lipofen was $0.4 million for the three months ended June 30, 2021, a decrease of $0.2 million compared to $0.6 million for the three months ended March 31, 2020.
Licensing revenue from the extended-release tramadol product (ConZip in the U.S. and Durela in Canada) was $0.05 million for the second quarter compared to $0.25 million for the three months ended June 30, 2020.
Product revenue increased by $1.3 million or 65% to $3.3 million for Q2 2021, compared to $1.9 million for the comparable period in 2020.
Product revenue from Epuris was $3.1 million for the quarter, an increase of $1.2 million from $1.9 million for the comparative period. According to IQVIA, Epuris had a prescription market share of approximately 43% in Canada for the three months ended June 30, 2021 compared to 41% for the three months ended June 30, 2020.
Product revenue for the remaining brands, Ozanex, Beteflam, Actikerall, Brinavess, Aggrastat and Vaniqa was $0.2 million, compared to $0.1 million for the three months ended June 30, 2020.
Total operating expenses increased to $2.3 million for the quarter compared to $2.1 million for Q2 2020. The increase in operating expenses is primarily due to the increase in Cost of Goods Sold.
Selling, general and administrative expense was $1.2 million for the quarter a decrease of 10% compared to $1.3 million for the three months ended June 30, 2020. The decrease in SG&A costs was driven by a decrease in costs related to legal and consulting spend.
Income from continuing operations was $2.8 million, or $0.11 per basic share in Q2 2021, compared to income from continuing operations of $0.4 million, or $0.02 per basic and diluted share in Q2 2020.
The Company had $16.1 (CDN$19.9) million in cash and no debt at June 30, 2021. The Company generated $7.6 million in cash from operating activities in the six month period ending June 30th, 2021 and used approximately $0.6 million in cash during the quarter for financing activities.
Subsequent to June 30, 2021, the Company assigned the office lease for its corporate operations head office to an arms' length third party. The term of the lease was 10 years and three months and commenced on January 1, 2019. The Company expects to incur a non-recurring early termination expense in the three-months ended September 30, 2021. It is expected that the early termination of the lease will result in net savings of approximately $25 thousand per month.
Financial Statements and MD&A
Cipher's Financial Statements for the quarter ended June 30, 2021 and Management's Discussion and Analysis (the "MD&A") for the three and six-months ended June 30, 2021 are available on the Company's website at www.cipherpharma.com in the "Investors" section under "Financial Reports" and on SEDAR at www.sedar.com.
Notice of Conference Call
Cipher will hold a conference call on August 13, 2021, at 8:30 a.m. (ET) to discuss its financial results and other corporate developments.
- To access the conference call by telephone, dial (416) 764-8688 or (888) 390-0546 and use conference ID 51158530.
- A live audio webcast will be available at https://produceredition.webcasts.com/starthere.jsp?ei=1487263&tp_key=bf24ee93ea
- An archived replay of the webcast will be available until August 20, 2021.
About Cipher Pharmaceuticals Inc.
Cipher Pharmaceuticals (TSX: CPH) is a specialty pharmaceutical company with a robust and diversified portfolio of commercial and early to late-stage products. Cipher acquires products that fulfill unmet medical needs, manages the required clinical development and regulatory approval process, and currently markets those products either directly in Canada or indirectly through partners in Canada, the U.S., and South America. For more information, visit www.cipherpharma.com.
Forward-Looking Statements
This document includes forward-looking statements within the meaning of applicable securities laws. These forward-looking statements include, among others, statements with respect to the impact of the Company's cost reduction plan, the potential for improved profitability of our hospital business, increased adoption of ABSORICA LD®, discussions with Galephar regarding new product opportunities, our objectives and goals and strategies to achieve those objectives and goals, as well as statements with respect to our beliefs, plans, expectations, anticipations, estimates and intentions. The words "may", "will", "could", "should", "would", "suspect", "outlook", "believe", "plan", "anticipate", "estimate", "expect", "intend", "forecast", "objective", "hope" and "continue" (or the negative thereof), and words and expressions of similar import, are intended to identify forward-looking statements.
By their very nature, forward-looking statements involve inherent risks and uncertainties, both general and specific, which give rise to the possibility that predictions, forecasts, projections and other forward-looking statements will not be achieved. Certain material factors or assumptions are applied in making forward-looking statements and actual results may differ materially from those expressed or implied in such statements. We caution readers not to place undue reliance on these statements as a number of important factors, many of which are beyond our control, could cause our actual results to differ materially from the beliefs, plans, objectives, expectations, anticipations, estimates and intentions expressed in such forward-looking statements. These factors include, but are not limited to, the extent and impact of the coronavirus (COVID-19) outbreak on our business including any impact on our contract manufacturers and other third party service providers, our ability to enter into development, manufacturing and marketing and distribution agreements with other pharmaceutical companies and keep such agreements in effect; our dependency on a limited number of products; our dependency on protection from patents that will expire; integration difficulties and other risks if we acquire or in-license technologies or product candidates; reliance on third parties for the marketing of certain products; the product approval process is highly unpredictable; the timing of completion of clinical trials, regulatory submissions and regulatory approvals; reliance on third parties to manufacture our products and events outside of our control that could adversely impact the ability of our manufacturing partners to supply products to meet our demands; we may be subject to future product liability claims; unexpected product safety or efficacy concerns may arise; we generate license revenue from a limited number of distribution and supply agreements; the pharmaceutical industry is highly competitive; requirements for additional capital to fund future operations; products in Canada may be subject to pricing regulation; dependence on key managerial personnel and external collaborators; no assurance that we will receive regulatory approvals in the U.S., Canada or any other jurisdictions and current uncertainty surrounding health care regulation in the U.S.; certain of our products are subject to regulation as controlled substances; limitations on reimbursement in the healthcare industry; limited reimbursement for products by government authorities and third-party payor policies; products may not be included on list of drugs approved for use in hospitals; hospital customers may make late payments or not make any payments; various laws pertaining to health care fraud and abuse; reliance on the success of strategic investments and partnerships; the publication of negative results of clinical trials; unpredictable development goals and projected time frames; rising insurance costs; ability to enforce covenants not to compete; risks associated with the industry in which we operate; we may be unsuccessful in evaluating material risks involved in completed and future acquisitions; we may be unable to identify, acquire or integrate acquisition targets successfully; legacy risks from operations conducted in the U.S.; inability to meet covenants under our long term debt arrangement; compliance with privacy and security regulation; our policies regarding returns, allowances and chargebacks may reduce revenues; certain current and future regulations could restrict our activities; additional regulatory burden and controls over financial reporting; reliance on third parties to perform certain services; general commercial litigation, class actions, other litigation claims and regulatory actions; the difficulty for shareholders to realize in the United States upon judgments of U.S. courts predicated upon civil liability of the Company and its directors and officers who are not residents of the United States; the potential violation of intellectual property rights of third parties; our efforts to obtain, protect or enforce our patents and other intellectual property rights related to our products; changes in U.S., Canadian or foreign patent laws; litigation in the pharmaceutical industry concerning the manufacture and supply of novel and generic versions of existing drugs; inability to protect our trademarks from infringement; shareholders may be further diluted if we issue securities to raise capital; volatility of our share price; the fact that we have a significant shareholder; we do not currently intend to pay dividends; our operating results may fluctuate significantly; and our debt obligations will have priority over the common shares of the Company in the event of a liquidation, dissolution or winding up.
We caution that the foregoing list of important factors that may affect future results is not exhaustive. When reviewing our forward-looking statements, investors and others should carefully consider the foregoing factors and other uncertainties and potential events. Additional information about factors that may cause actual results to differ materially from expectations, and about material factors or assumptions applied in making forward-looking statements, may be found in the "Risk Factors" section of the Company's Annual Information Form for the year ended December 31, 2020, and elsewhere in our filings with Canadian securities regulators. Except as required by Canadian securities law, we do not undertake to update any forward-looking statements, whether written or oral, that may be made from time to time by us or on our behalf; such statements speak only as of the date made. The forward-looking statements included herein are expressly qualified in their entirety by this cautionary language.
1) | At the CAD/USD exchange rate June 30, 2021 |
2) | EBITDA is a non-IFRS financial measure. The term EBITDA (earnings before interest, taxes, depreciation and amortization,) does not have any standardized meaning under IFRS and therefore may not be comparable to similar measures presented by other companies. Rather, these measures are provided as additional information to complement IFRS measures by providing a further understanding of operations from management's perspective. The Company defines Adjusted EBITDA as earnings before interest expense, income taxes, depreciation of property and equipment, amortization of intangible assets, loss on debt extinguishment, non-cash share-based compensation, changes in fair value of derivative financial instruments, impairment of intangible assets and goodwill and foreign exchange gains and losses from the translation of Canadian cash balances. |
The Following is a summary of how EBITDA and Adjusted EBITDA are calculated:
SOURCE Cipher Pharmaceuticals Inc.